Usp Reference Standard Catalogue
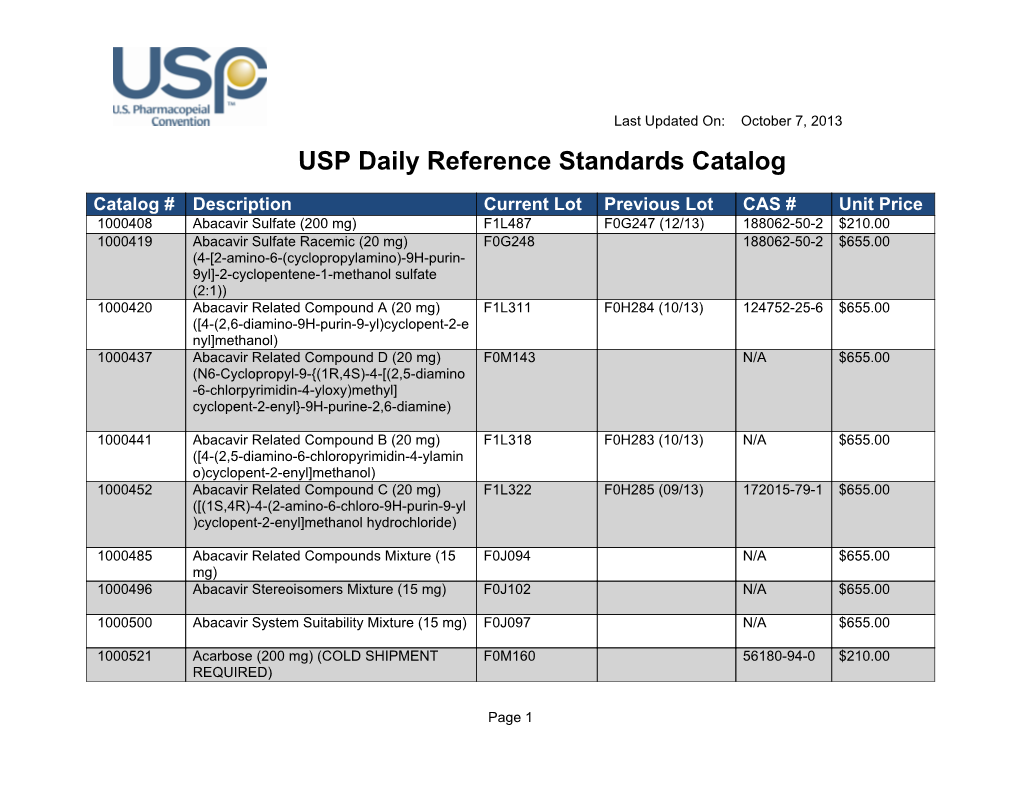
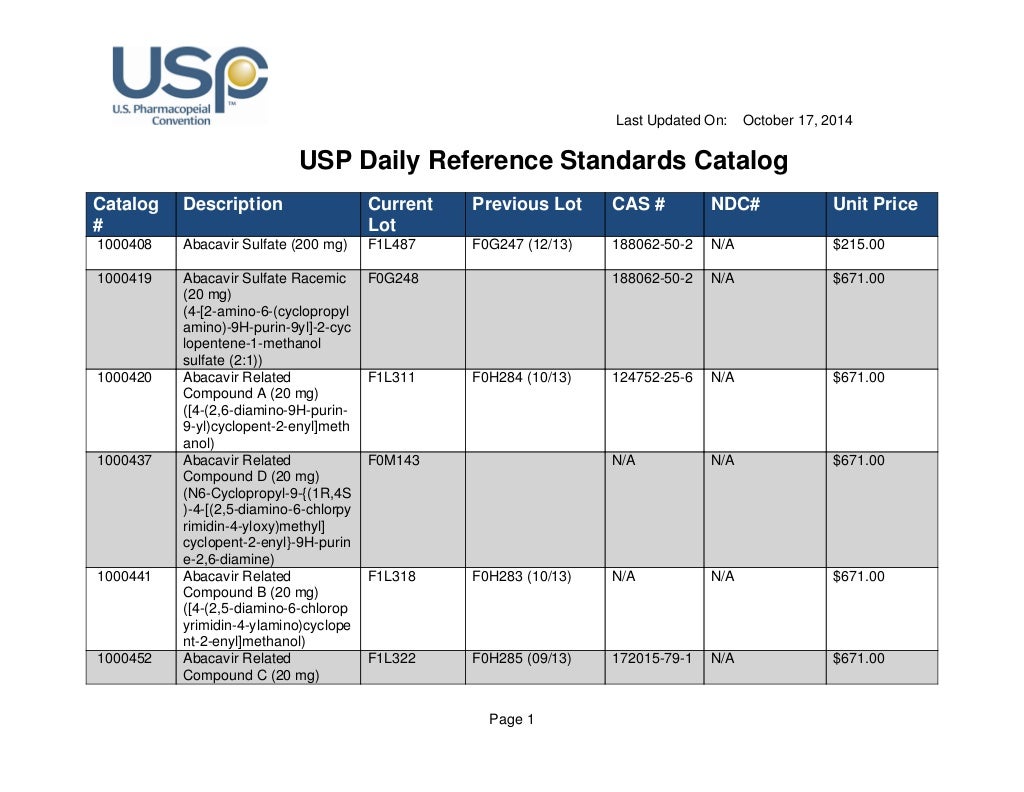
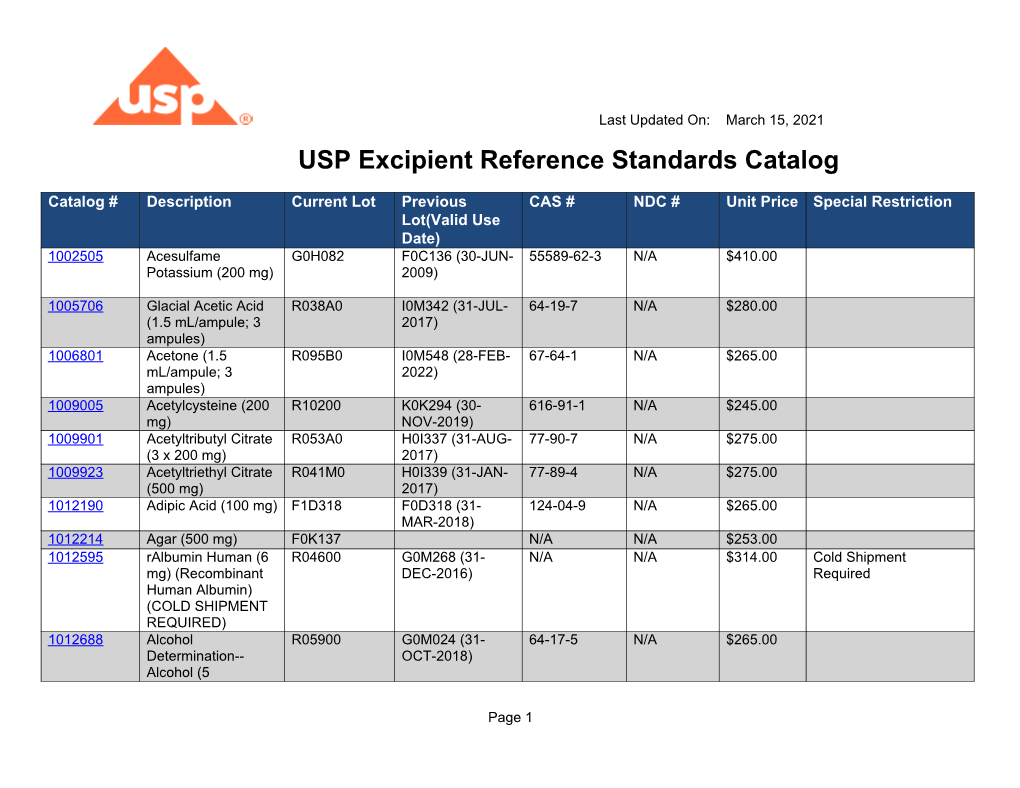
Usp Reference Standard Catalogue - Learn how usp develops and releases reference standards for analytical, clinical, pharmaceutical, and research laboratories. To help users of usp reference standards locate and apply materials relevant to their work, usp offers these convenient tools and resources: Find out what's new at usp, including reference standards, monographs, impurities, and more. Download the catalog, access the. See the list of newly available and unavailable rs as of april 28,. Usp reference standards are substances of high purity and critical characteristics used for pharmacopeial assays and tests. Find out how to order, prepare and use usp rs's. A pdf document that lists the usp reference standards for dietary supplements, including their names, codes, cas numbers, and prices. Individual sds can be downloaded, saved, and printed. See the tables for new, existing, and backordered rs lots, and the. In a xml format please note that you can download the terms and conditions of. Usp offers over 7,000 highly characterized control samples of drug substances, excipients, food ingredients, and more for quality testing. This reference standard (rs) supports general chapter dissolution. Usp reference standards are substances of high purity and critical characteristics used for pharmacopeial assays and tests. A pdf document that lists the usp reference standards for dietary supplements, including their names, codes, cas numbers, and prices. The document covers the scope, process,. Download the catalog, access the. Learn how usp develops and releases reference standards for analytical, clinical, pharmaceutical, and research laboratories. Learn how usp reference standards are linked to. Individual sds can be downloaded, saved, and printed. To help users of usp reference standards locate and apply materials relevant to their work, usp offers these convenient tools and resources: See the tables for new, existing, and backordered rs lots, and the. Learn about the uses, development, availability and validity of usp reference standards, which are intended for test and assay use only. In a xml format please. A pdf document that lists the usp reference standards for dietary supplements, including their names, codes, cas numbers, and prices. This reference standard (rs) supports general chapter dissolution. Usp offers more than 3,500 highly characterized specimens of drug substances, excipients, food ingredients, and other compounds for analytical testing. Usp reference standards are substances of high purity and critical characteristics used. Live microbial products (lmps) can exist in multiple regulatory categories, as drugs/biologics (live biotherapeutic products), dietary supplements and foods (commonly called probiotics), and. See the list of newly available and unavailable rs as of april 28,. The official catalog of reference standards and authentic. Search by reference standard name, catalog number, or cas number. Learn about the uses, development, availability. Learn about the uses, development, availability and validity of usp reference standards, which are intended for test and assay use only. Browse the quality solution sheets (qss) to access all the relevant. In a xml format please note that you can download the terms and conditions of. Find out how to order, prepare and use usp rs's. Live microbial products. To help users of usp reference standards locate and apply materials relevant to their work, usp offers these convenient tools and resources: Live microbial products (lmps) can exist in multiple regulatory categories, as drugs/biologics (live biotherapeutic products), dietary supplements and foods (commonly called probiotics), and. The document covers the scope, process,. The official catalog of reference standards and authentic. Search. Learn about usp, its documentary and physical standards, and how. Search by reference standard name, catalog number, or cas number. Find out what's new at usp, including reference standards, monographs, impurities, and more. Browse the quality solution sheets (qss) to access all the relevant. You can also download the european pharmacopoeia daily reference standards catalogue: The catalog covers a wide range of ingredients,. Usp offers more than 3,500 highly characterized specimens of drug substances, excipients, food ingredients, and other compounds for analytical testing. Learn about usp, its documentary and physical standards, and how. Usp offers over 7,000 highly characterized control samples of drug substances, excipients, food ingredients, and more for quality testing. To help users. This reference standard (rs) supports general chapter dissolution. The document covers the scope, process,. See the tables for new, existing, and backordered rs lots, and the. Usp offers over 7,000 highly characterized control samples of drug substances, excipients, food ingredients, and more for quality testing. Find the latest information on usp reference standards (rs) availability, validity, and changes for the. Search by reference standard name, catalog number, or cas number. Find out how to order, prepare and use usp rs's. A pdf document that lists the usp reference standards for dietary supplements, including their names, codes, cas numbers, and prices. In a xml format please note that you can download the terms and conditions of. Find the latest information on. Find out what's new at usp, including reference standards, monographs, impurities, and more. A pdf document that lists the usp reference standards for dietary supplements, including their names, codes, cas numbers, and prices. Below are several tables with the latest usp reference standard (rs) information for the month of may (2020). Find and buy usp reference standards for testing drug. Usp reference standards are substances of high purity and critical characteristics used for pharmacopeial assays and tests. Below are several tables with the latest usp reference standard (rs) information for the month of may (2020). Learn about the uses, development, availability and validity of usp reference standards, which are intended for test and assay use only. Learn about usp, its documentary and physical standards, and how. You can also download the european pharmacopoeia daily reference standards catalogue: In a xml format please note that you can download the terms and conditions of. This reference standard (rs) supports general chapter dissolution. The document covers the scope, process,. Find out how to order, prepare and use usp rs's. See the tables for new, existing, and backordered rs lots, and the. Learn how usp develops and releases reference standards for analytical, clinical, pharmaceutical, and research laboratories. Browse the quality solution sheets (qss) to access all the relevant. A pdf document that lists the usp reference standards for dietary supplements, including their names, codes, cas numbers, and prices. Download the catalog, access the. Individual sds can be downloaded, saved, and printed. Find out what's new at usp, including reference standards, monographs, impurities, and more.USP Daily Reference Standards Catalog DocsLib
Usp daily reference standards catalog
Usp daily reference standards catalog
Official Usp Reference Standards PDF Metrology Calibration
USP Reference Standards Catalog DocsLib
USP Reference Standards Catalog Last Updated On November 7, 2020
USP標準品|日本バリデーション・テクノロジーズ
USP Excipient Reference Standards Catalog DocsLib
USP Dietary Supplements Reference Standards Catalog PDF Magnesium
11 USP Reference Standards PDF Metrology Calibration
Learn How Usp Reference Standards Are Linked To.
Usp Offers Over 7,000 Highly Characterized Control Samples Of Drug Substances, Excipients, Food Ingredients, And More For Quality Testing.
Find The Latest Information On Usp Reference Standards (Rs) Availability, Validity, And Changes For The Month Of June 2020.
See The List Of Newly Available And Unavailable Rs As Of April 28,.
Related Post: